textile-based electrochemical sensors

back in 2016, I started a project to see if I could create electrochemical sensors that were flexible and fabricated as (or printed on) textiles. this exploration continued right up until COVID hit, and I've never really sat down to document what I did over the years.
At the time I started, I had a background in analytical electrochemistry from doing research with the University of Florida's bioengineering department in college (shout out to my mentor, Dr. Eric McLamore). My main motivation was to design a wearable sensor that could be used non-invasively to detect various small molecules in fluids such as sweat.
My main takeaway from this exercise is that building anything that goes off the path of conventional electronics is hard. These explorations were able to produce some actual working sensors, but none with the fine-grained resolution necessary to detect small molecules (such as glucose or alcohol) at the concentrations present in skin-based fluids. Nevertheless, I learned a lot in the process and think it's worth detailing the journey.
There were several approaches I explored:
- laser-inscribed graphene (LIG) etched onto textiles
- metallic threads knitted into a textile
- embroidered metallic threads
- metallic & graphene-based ink screen-printed onto textiles
Below is a high-level overview on each of these explorations.
laser-inscribed graphene

believe it or not, it is possible to create useful graphene-based materials with (mostly) household supplies. Using a hobby-grade laser etcher and some Kapton tape, one can generate graphene by running the laser etcher over the Kapton tape, which carbonizes the polyamide in the tape and creates a thin film of graphene oxide.
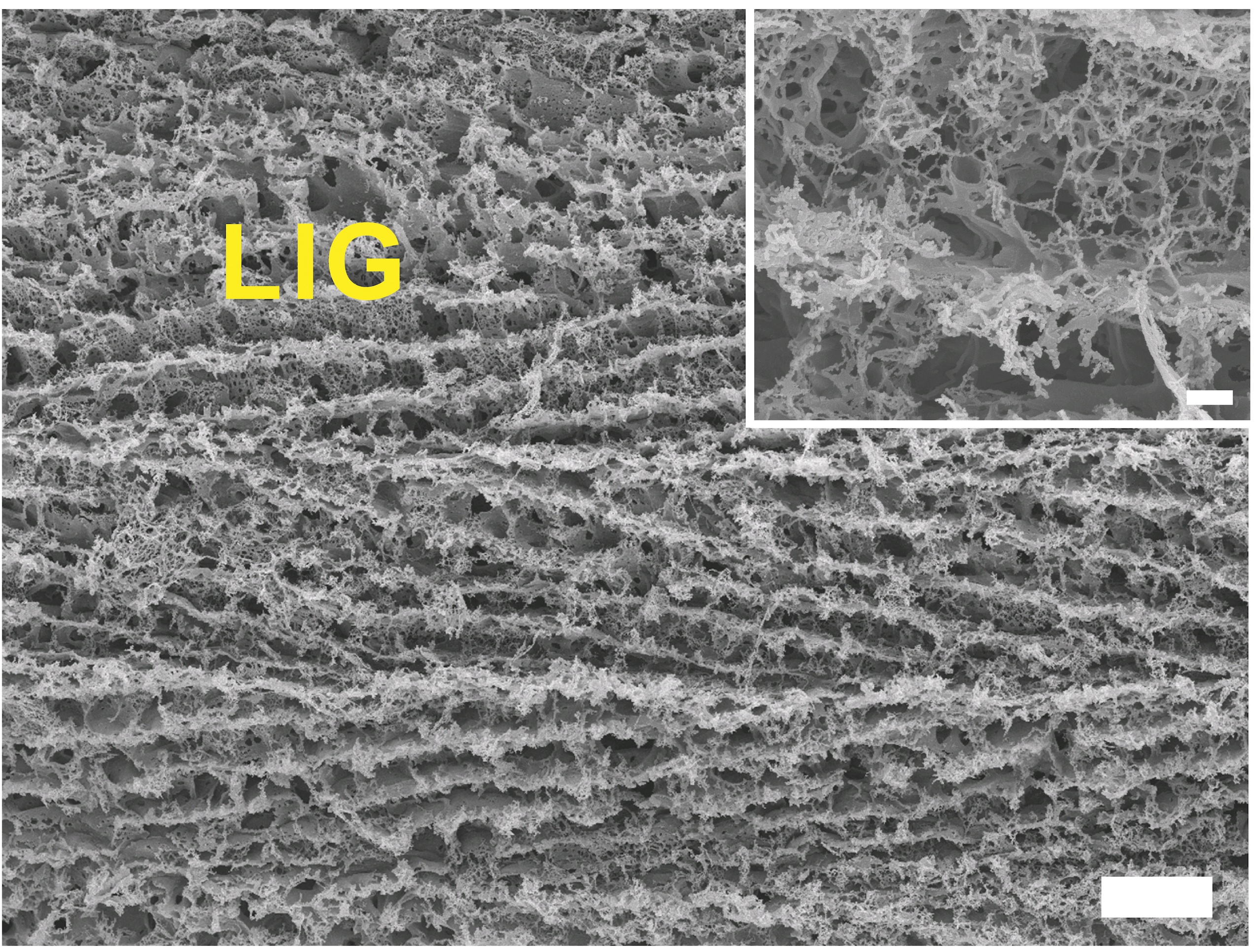
It should be pointed out that graphene oxide is not pure graphene, as it isn't a single sheet of one atom-layer thick carbon, but rather a two-dimensional stack of carbon sheets with oxygentated groups that produce “kinks” at the ends of the sheets, resulting in something that looks roughly like this under a scanning electron microscope:
Graphene oxide is a useful material for electrochemical applications: stacked, imperfect graphene sheets contribute to a larger electroactive surface area (ESA), as the complexity of the emergent structure provides more room for electrons to be exchanged at the material’s surface. Here's a photo from Rice University of laser-inscribed graphene under a scanning electron microscope (SEM):
subsequent tests I ran on these electrodes did confirm that the material does have a reasonable ESA, especially after decorating the surface with metal nanoparticles. with a technique referred to as cyclic voltammetry, we can see a material's electron transfer characteristics and calculate the ESA (roughly the area under the peaks). with these electrodes, we see a large jump in ESA after decorating the graphene with platinum nanoparticles:

however, etching the material directly onto a fabric was quite brittle. these electrodes did not handle mechanical stress well - they lost conductivity often after any sort of bending. none of the underlying textiles used were stretchable, as a fairly sturdy construction (aka woven) was needed to laser etch the Kapton tape on top of.
I also performed "platform stability" tests, where I ran CV scans hundreds of times using the same sensor, and the signal degraded with each scan to the point of being unusable. So, I started to look at alternative methods to fabricate textile-based sensors.
knitted metallic threads
another approach I explored was using metal-coated yarns knitted into a textile structure. the thinking here was that knitted structures are more pliable and conformant to someone’s body. they have the ability to stretch and deform, but then return back to their original shape.
being in New York City at the time, I was able to pique the interest of knitting machine manufacturer Shima Seiki to fabricate electrode patterns designed with their CAD software and machines.
The samples produced use a silver-coated nylon yarn, which is manufactured mostly for EMF shielding and anti-microbial applications. You may have clothing with these yarns if you’ve ever purchased sportswear from companies like Lulelemon.

While these samples did bend and stretch quite well, upon testing it became clear that the electrical signal produced by these electrodes was extremely noisy. In hindsight, it makes a ton of sense — the “wires” produced by knitted structure do not add up to a discrete, straightforward path for electrons to flow. Rather, they are a complex set of interconnects as the yarn is looped upon itself, causing electrons to scatter more stochastically, which degrades the fidelity of the signal.
Furthermore, the costs associated with knitting manufacturing are quite high. I ultimately decided to move on from this approach, mainly due to the noise making knit samples unusable.
embroidered metallic threads
While in the process of exploring knitted sensors, I came across a supplier with a process for coating threads with gold and ordered some yarn, originally with the intention to test against knitted samples.
The yarns were instead repurposed as electrodes embroidered into a fabric. Two main factors led to this decision:
- the initial experiments with knitting went poorly
- the fibers composing the yarn were too fine and the gold flaked off during processing to the point that the yarn was no longer conductive
Brainstorming at home, my mom helpfully suggested to twist the yarns into something thicker, similar to threads that would be used for embroidery:

the electrodes created using gold plated yarns produced the highest fidelity signal and largest ESA out of all fabrication methods attempted, as shown by this cyclic voltammogram:

With the ESA evaluated, I began to prepare samples for detection of hydrogen peroxide.
Why hydrogen peroxide? The enzymes glucose oxidase and alcohol oxidase both catalyze reactions that produce hydrogen peroxide as a by-product. So by testing a sensor’s ability to detect H2O2, we are indirectly able to characterize its ability to detect glucose or alcohol, as it is possible to place these enzymes on an electrode surface to produce H2O2.
Preparation of the samples involved running an electrochemical deposition process to add Prussian blue and gold nano-particles to the electrode surface. Once this process was complete, it was possible to start testing hydrogen peroxide detection.
here’s a chart showing the (mostly) linear response in current for increasing concentrations of hydrogen peroxide:

After performing these tests, I paused on this project for a couple years to focus on my career in software development.
screen-printed textile electrodes
In 2019, I started my first remote job working for a Bay Area based startup and suddenly found myself with more free time to engage in side-projects.
So I decided to dust off my notes from previous attempts at sensor fabrication and decided to take a more tried-and-true approach to electrochemical sensor manufacturing: screen-printing.
Screen-printing is a surprisingly versatile process. While more well known for making graphic tees and posters, screen-printing is also responsible for the glucose test strips diabetic patients use and other electronics such as membrane switches.
I had already identified several vendors for printable conductive silver and graphene-based inks from prior research. Where I wanted to invest time was in creating patterns that would work well for applications requiring wires that can withstand significant mechanical stress.
Inspired by a research paper on fractal designs for stretchable electronics, I wrote a piece of software (which was aptly named CurveTool) to help me generate space-filling, serpentine patterns in a vector-based format I could further process in Adobe Illustrator.
You can generate these designs yourself - just click any two adjacent squares in the grid below to get started:
Once I had the patterns generated, I spent time learning how to prepare masks to start printing.
These masks are created by exposing a UV sensitive film to UV light, with the design laying between the UV light and the photosensitive film to block light selectively where the design is. The film hardens wherever light hits. Then, you can wash away the non-hardened parts of the film to expose the design, which allows you to push paint through the screen and onto whatever material you’re printing on.

This took lots of trial and error — my initial designs weren’t printed in black enough ink, so the film would harden even where the design was present, and this only became apparent after doing test prints where ink wouldn’t fully pass through the screen:

Eventually though, I got the hang of mask prep and was able to print successfully. With the base silver layer printed, I started to think about what materials would be suitable for the working electrode (aka the one performing the detection).
I tested multiple options:
- conductive ink from a vendor made out of graphite and prussian blue. specifically designed for hydrogen peroxide detection, but not optimized for flexible / stretchable applications.
- home-grown conductive ink from a blend of LIG and an elastomeric polymer.

Unfortunately, this is where the story comes to a somewhat abrupt end. I was starting to perform these just before the COVID pandemic started – the data I gathered was around February / March 2020. I was not able to identify a working electrode material that would have suited this application. The data gathered is honestly not interesting enough to share. Once everything shut down, I didn't have as many avenues to explore other options with different vendors. I did, however, get this fun photo of the ability to stretch / bend the electrodes with no impact to their conductivity:

wrapping up
And so ends this tale of trying to build textile-based electrochemical sensors. The developments I was working on in 2020 were somewhat arrested after March – it was hard to keep momentum up after a good chunk of the world shut down. I was also laid off from my job a couple months later which didn't help much with motivation. Post-COVID, life took me in different directions and I decided to end any further work in this space.
While not the most satisfying ending, I learned a lot of interesting things about manufacturing in general and was exposed to areas of tech that I never would have as someone who works in software, such as textile engineering. All in all, I'm just happy to finally be sharing some of the work that I did.
Feel free to drop a line if you're ever wanting to geek out on electrochemistry or ideating on new categories in the wearables space. If you made it this far, congratulations on putting up with my writing! And thanks for reading.